Error
This video doesn’t exist
Bristol-Myers Squibb’s ORENCIA® (abatacept) is Approved by European Commission for the Treatment of Adult Patients with Rheumatoid Arthritis

September 6, 2016
Bristol-Myers Squibb Company has announced that the European Commission has approved ORENCIA ® (abatacept) intravenous (IV) infusion and subcutaneous (SC) injection, combined with methotrexate (MTX), for treating highly active and progressive disease in adult patients with rheumatoid arthritis (RA) not previously treated with MTX. Through this approval, ORENCIA has turned out as the first biologic therapy with an indication in the European Union (EU) specifically applicable to the treatment of MTX-naive RA patients with highly active and progressive disease. This approval permits the expanded marketing of ORENCIA in all 28 Member States of the EU.
FDA Accepts Supplemental Biologics License Application for Merck’s KEYTRUDA® (pembrolizumab) for First-Line Treatment of Patients with Advanced Non-Small Cell Lung Cancer

September 7, 2016
Merck has announced that the U.S. Food and Drug Administration (FDA) has accepted the supplemental Biologics License Application (sBLA) for KEYTRUDA® (pembrolizumab) for Priority Review. KEYTRUDA® is Merck’s anti-PD-1 therapy that is used as the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC). In addition, the FDA granted Breakthrough Therapy Designation for this indication. Merck has also submitted a Marketing Authorization Application to the European Medicines Agency for KEYTRUDA®.
Janssen Submits Marketing Authorisation Application to European Medicines Agency for Darunavir-Based Single Tablet Regimen for the Treatment of HIV-1

September 12, 2016
Janssen-Cilag International NV (Janssen) has announced that it has submitted a Marketing Authorisation Application to the European Medicines Agency (EMA), which seeks approval for a new once-daily darunavir-based single tablet regimen (STR). This tablet would be the first protease inhibitor (PI)-based STR option (D/C/F/TAF FDC) if approved, indicated as a complete regimen for the treatment of human immunodeficiency virus type 1 (HIV‑1) infection in adults and adolescents (aged 12 years and older with body weight of at least 40 kg).
Janssen submits application to European Union seeking approval of sirukumab for rheumatoid arthritis

September 12, 2016
Janssen-Cilag International NV (Janssen) has announced the submission of a Marketing Authorisation Application (MAA) to the European Medicines Agency (EMA) seeking approval of sirukumab for treating adult patients with moderately to severely active rheumatoid arthritis (RA). Approximately 6.2 million Europeans are affected by RA, which is a chronic, systemic inflammatory condition. Sirukumab is a human monoclonal IgG1 kappa antibody targeting the cytokine IL-6, a naturally occurring protein that is believed to play a key role in autoimmune conditions like RA.
Merck and Pfizer Announce Investigational Ertugliflozin Met Primary Endpoint of A1C Reduction When Added to Metformin and Sitagliptin for the Treatment of Type 2 Diabetes

September 15, 2016
Merck, in partnership with Pfizer Inc., has announced that a Phase 3 study (VERTIS SITA2) of ertugliflozin, an investigational oral SGLT2 inhibitor for the treatment of patients with type 2 diabetes, met its primary endpoint. Both 15 mg and 5 mg daily doses of ertugliflozin revealed meaningfully greater reductions in A1C of 0.69 percent and 0.76 percent, respectively, in comparison with placebo (p<0.001, for both comparisons), when added to patients on a background of stable metformin (≥1500 mg/day) and sitagliptin (100 mg/day). For the first time, these study results were presented during an oral session at the 52nd Annual Meeting of the European Association for the Study of Diabetes (EASD) in Munich, Germany.
Phase III combination trial of Bydureon and Forxiga shows significant benefits in patients with type-2 diabetes

September 16, 2016
Positive results from the Phase III DURATION-8 trial confirmed that Bydureon (exenatide extended-release formulation) 2mg once weekly, combining with Forxiga (dapagliflozin) 10mg once daily significantly reduced blood sugar as measured by HbA1c, versus the individual medicines alone in patients with type-2 diabetes ineffectively controlled on metformin. The results were presented today at the 52nd Annual Meeting of the European Association for the Study of Diabetes (EASD) in Munich, Germany, and simultaneously published in the journal ‘The Lancet Diabetes & Endocrinology’.
Pfizer Gets Positive CHMP Opinion for IBRANCE® (palbociclib) in Combination with Endocrine Therapy for The Treatment of HR+/HER2- Metastatic Breast Cancer in Europe

September 16, 2016
Pfizer Inc. has announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion recommending that IBRANCE® (palbociclib) be granted marketing authorization in the European Union (EU) for treating women with hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2-) locally advanced or metastatic breast cancer. This positive opinion from CHMP is for IBRANCE to be used in combination with fulvestrant in women who have received prior endocrine therapy, as well as in combination with an aromatase inhibitor. European Commission (EC) will now review The CHMP’s opinion.
U.S. FDA Accepts Biologics License Application for Romosozumab, Amgen and UCB Announce

September 26, 2016
Amgen and UCB have announced that the U.S. Food and Drug Administration (FDA) has accepted for review the Biologics License Application (BLA) for romosozumab. It is an investigational monoclonal antibody for treating osteoporosis in postmenopausal women at increased risk of fracture. Romosozumab works by binding and hindering the activity of the protein sclerostin, naturally occurring in the bone, thereby reducing bone resorption and increasing bone formation.
Amgen Announces Top-Line Results from Phase 3 KYPROLIS® (Carfilzomib) CLARION Study in Patients with Newly Diagnosed Multiple Myeloma

September 27, 2016
Amgen has announced top-line results of the Phase 3 CLARION trial evaluating an investigational regimen of KYPROLIS® (carfilzomib), melphalan and prednisone (KMP) versus Velcade® (bortezomib), melphalan and prednisone (VMP) for 54 weeks in patients with newly diagnosed multiple myeloma who were not eligible for hematopoietic stem-cell transplant. The trial did not meet the primary endpoint of superiority in progression-free survival (PFS). The observed hazard ratio (KMP versus VMP) was 1.21 (95 percent CI, 0.90 – 1.64) while the data for overall survival, a secondary endpoint, are not yet mature. Neither result was statistically significant. These data will be submitted to a future medical conference and for publication.
Two Cardiovascular Collaborations Announced by Amgen and Arrowhead Pharmaceuticals

September 29, 2016
Amgen and Arrowhead Pharmaceuticals Inc. have announced two license and collaboration agreements to develop and commercialize RNA interference (RNAi) therapies for cardiovascular disease. Under the first agreement, Amgen receives a worldwide, exclusive license to Arrowhead’s novel, RNAi ARC-LPA program. The RNAi molecules are designed to decrease elevated lipoprotein, which is a genetically validated, independent risk factor for atherosclerotic cardiovascular disease. As per the second agreement, Amgen receives an option to a worldwide, exclusive license for a RNAi therapy for an undisclosed genetically validated cardiovascular target. Amgen will be wholly responsible for clinical development and commercialization in both agreements.
Treatment outcome data from The Prostate Cancer Registry presented for the first time at the 2016 ESMO Congress

October 10, 2016
Janssen-Cilag International NV has presented the first reported primary treatment outcome data from The Prostate Cancer Registry at the 2016 European Society for Medical Oncology (ESMO) Congress in Copenhagen, Denmark. The Prostate Cancer Registry is the first and largest prospective study of men with metastatic castration-resistant prostate cancer (mCRPC) in Europe. The preliminary data suggest that chemotherapy-naïve patients benefit more from treatment than post-chemotherapy patients. Furthermore, patients have a higher prostate-specific antigen (PSA) response when treated with androgen receptor-targeted agents than with taxanes, after first line docetaxel treatment.
Pfizer Presented New Data on XELJANZ® for Ulcerative Colitis at UEG Week 2016

October 15, 2016
Pfizer Inc. announced that three abstracts for XELJANZ® (tofacitinib citrate), being investigated in moderate to severe ulcerative colitis (UC), were presented at the United European Gastroenterology Week (UEG Week 2016), October 15-19 in Vienna, Austria. The tofacitinib presentations highlighted new research results from the Phase 3 Oral Clinical Trials for tofAcitinib in ulceratiVE colitis (OCTAVE) Induction trials, including one oral presentation looking at the impact of prior treatment with tumor necrosis factor inhibitors (TNFi) on efficacy endpoints. Furthermore, two abstracts were accepted as poster presentations, highlighting results by endoscopic response, and onset of action, respectively.
Amgen Announces Positive Top-Line Results From XGEVA® (Denosumab) Phase 3 Trial for Patients with Multiple Myeloma

October 20, 2016
Amgen has announced that a Phase 3 study evaluating XGEVA® (denosumab) versus zoledronic acid met the primary endpoint of non-inferiority (hazard ratio = 0.98, 95 percent CI, 0.85 – 1.14) in delaying the time to first on-study skeletal-related event (SRE) in patients with multiple myeloma. The secondary endpoints of superiority in delaying time to first SRE and delaying time to first-and-subsequent SRE were not met. XGEVA’s hazard ratio versus zoledronic acid for overall survival was 0.90 (95 percent CI, 0.70 – 1.16).
NICE Recommends Oral OTEZLA® (Apremilast) for Adults with Chronic Plaque Psoriasis

October 20, 2016
Celgene has announced that adult patients in England and Wales with chronic plaque psoriasis will now have access to oral OTEZLA ® (apremilast) after a positive final appraisal determination from the National Institute for Health and Care Excellence (NICE). The decision is the conclusion of a NICE Rapid Review. It ensures patients in England and Wales will join those in Scotland, who have been benefitting from access to OTEZLA since Scottish Medicines Consortium (SMC) recommended it in June 2015. It has been estimated that Psoriasis has affected around 960,000 adults in the UK, seriously impacting their daily lives.



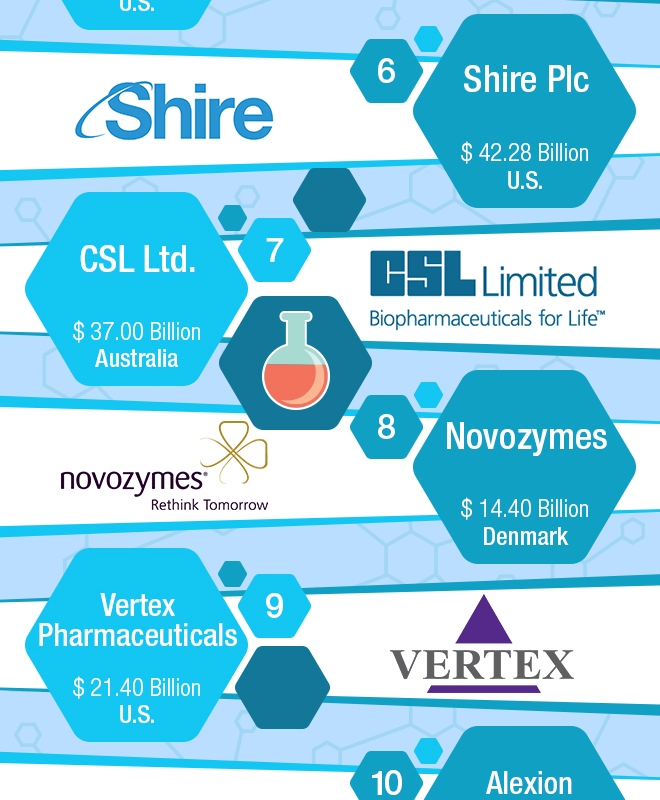
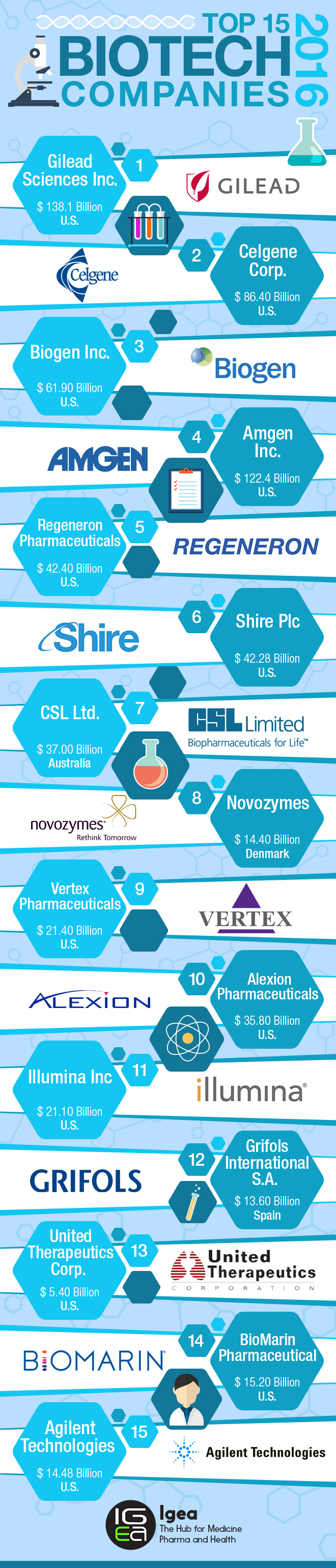
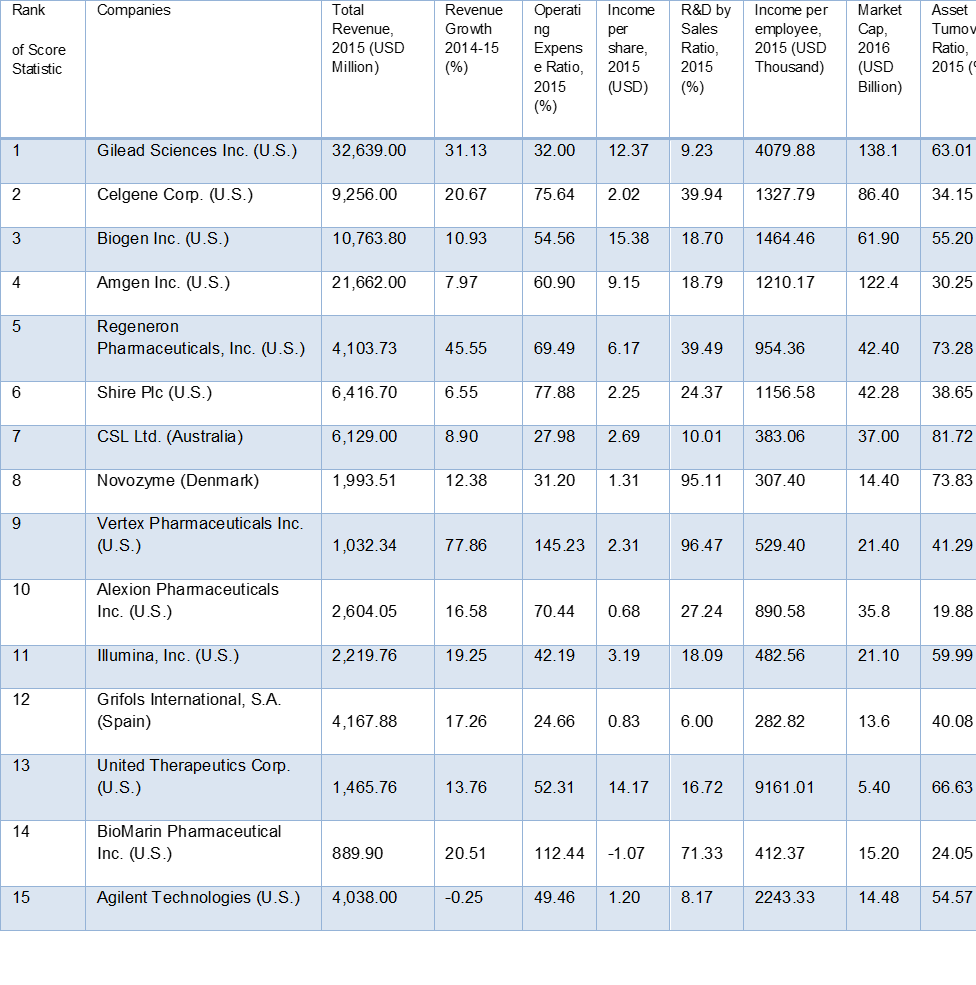 Sources: SEC filings, annual reports, Genetic Engineering & Biotechnology News and Forbes Media.
Sources: SEC filings, annual reports, Genetic Engineering & Biotechnology News and Forbes Media.
























![pfizer_rgb_pos_canvassed[1]](https://igeaweb.wordpress.com/wp-content/uploads/2016/04/pfizer_rgb_pos_canvassed1.jpg?w=161&h=121)




